Mechanism of Disease
Atopic dermatitis (AD) is as complex skin disease that can impact patients
AD is a heterogeneous, chronic, inflammatory skin disease that can significantly impact patients’ quality of life. Abnormalities in genetics, immune system and skin barrier responses, microbiome disturbance, and other environmental factors are all thought to play a part in the development of the disease. The molecular heterogeneity of AD is not fully elucidated and continues to be investigated; the pathogenesis is not limited to a single molecular entity or pathway especially as the disease progresses.1,2
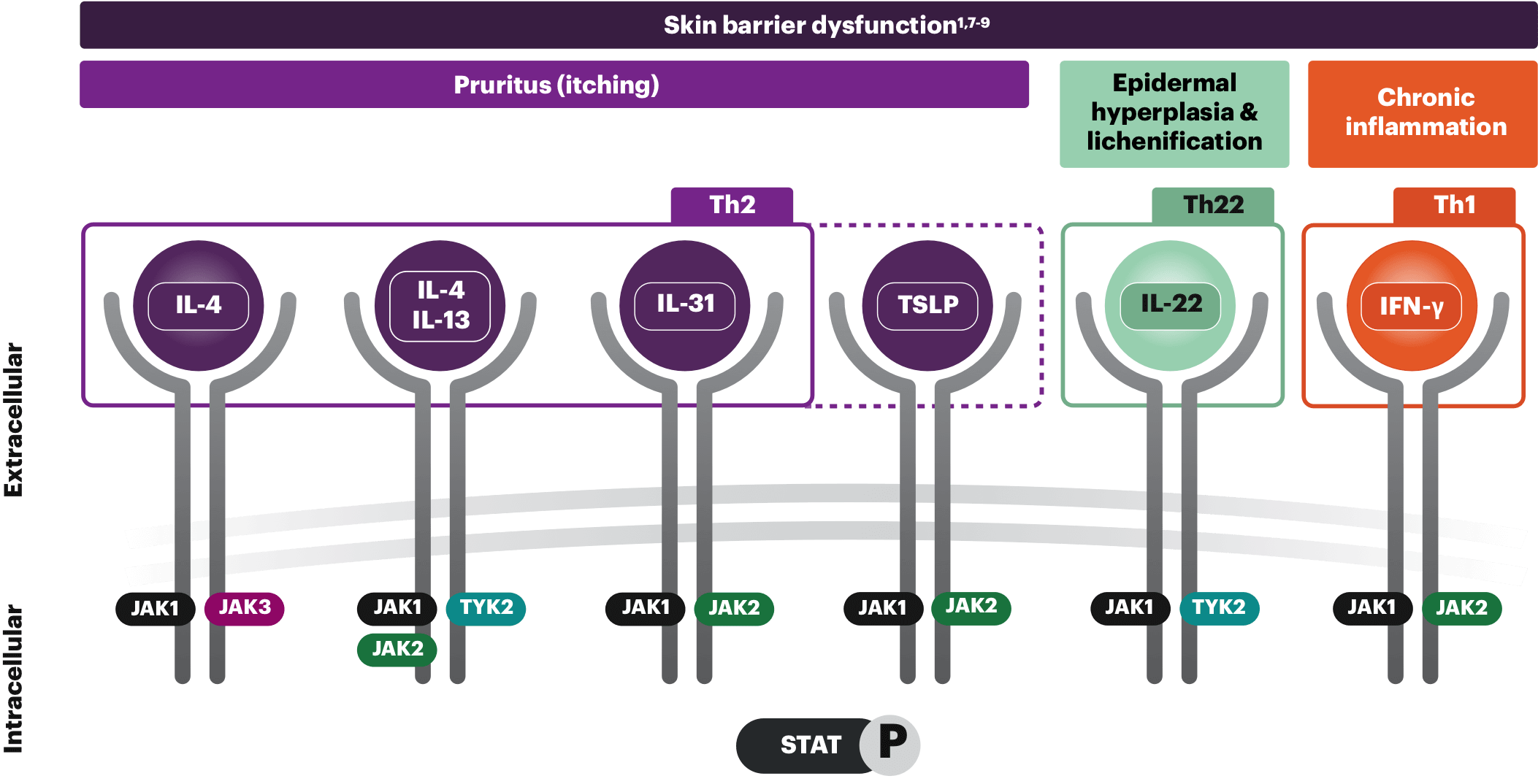
Exogenous triggers (eg, allergens and microbes) and endogenous triggers (eg, skin barrier dysfunction) result in keratinocytes expression of cytokines—such as thymic stromal lymphopoietin (TSLP), IL-33, and IL-25—which activate Th2 immune cells, including Langerhans cells and dermal dendritic cells to produce additional Th2 cytokines.1,3-6
These cells are thought to be activated and migrate to regional lymph nodes where they initiate and amplify the abnormal immune response which causes inflammation, skin barrier disruption, activation and growth of sensory neurons, itch and pain that is associated with AD. While the causes of epidermal barrier disruption are complex and not yet fully understood, the Th2 cytokines, IL-4 and IL-13, are thought to play an important role.1-5
These cytokines as well as IL-22, IL-31, TSLP, and IFN-γ interact with the intracellular Janus kinase (JAK)-signal transducer and activator transcription (STAT) pathway. Dysregulation of the JAK-STAT pathway is thought to contribute to inflammatory responses that is associated in AD.1,5
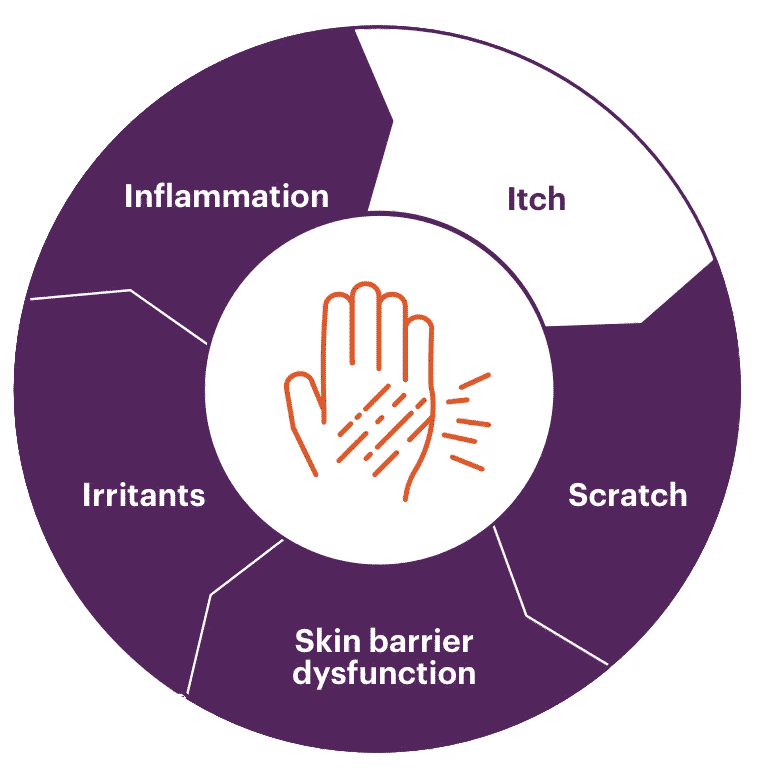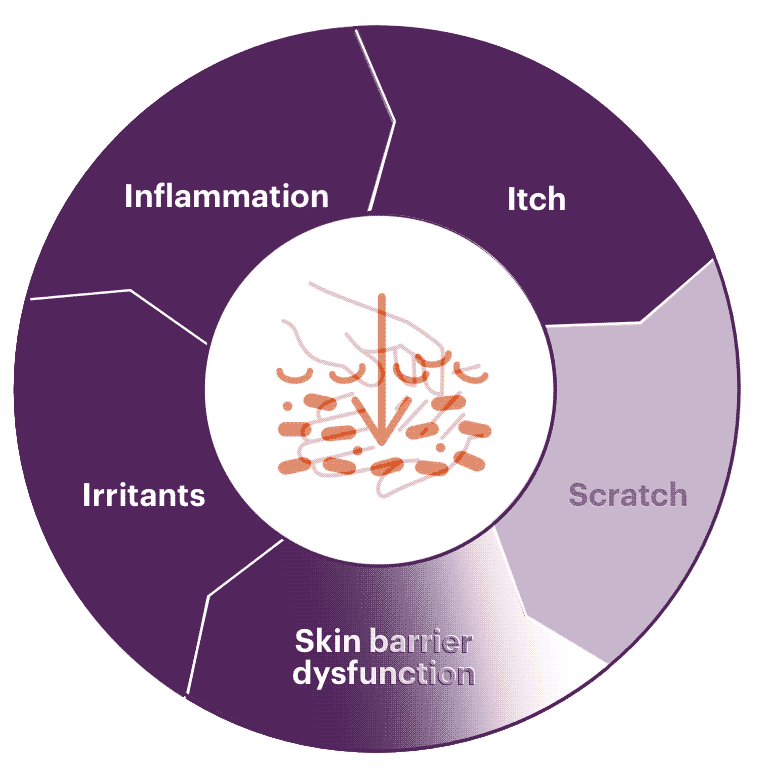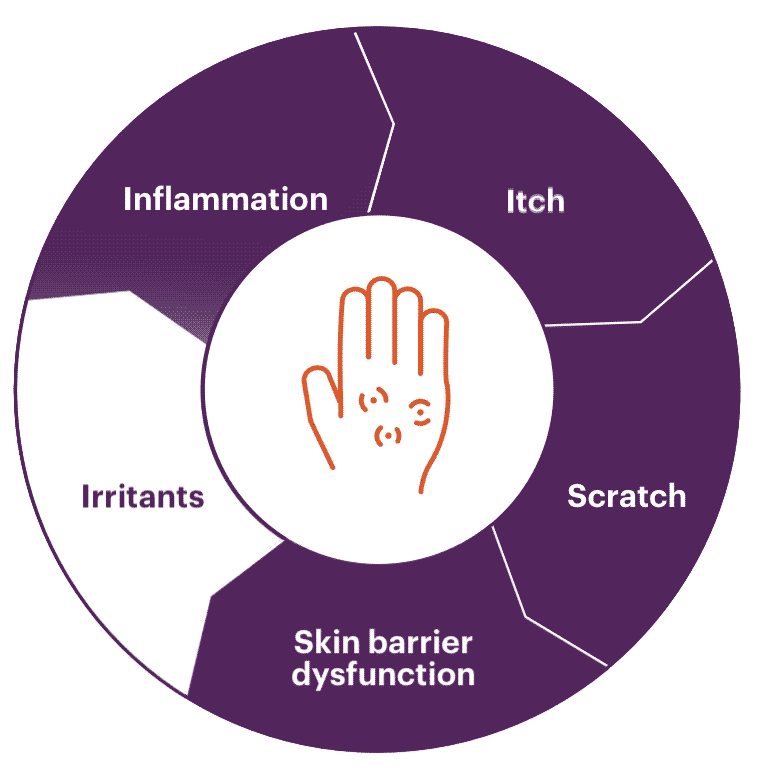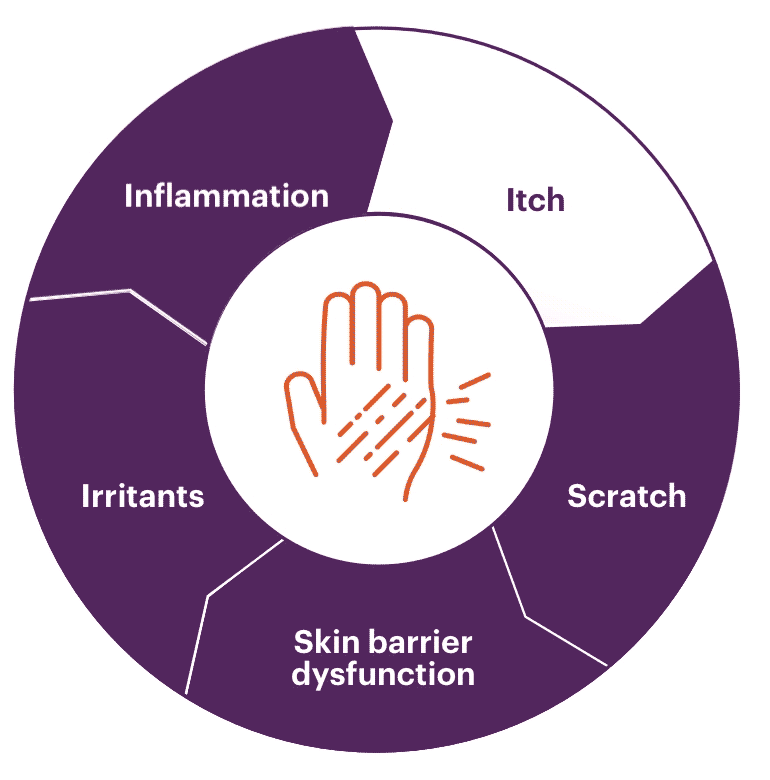
AD is complex and multifactorial
The JAK-STAT pathway, one of the pathways involved in AD’s complex pathophysiology, transmits signals from cytokines. These signals contribute to the overproduction of proinflammatory cytokines and exacerbate skin inflammation and itch.1 Select an area below to explore more.
The clinical impact of these pathways on patients with AD is not fully understood.
Th2, Th22, and Th1 cytokines shown are not exhaustive; other cytokines in the Th2, Th22, and Th1 classes are not shown and there is increasing attention regarding endotype presentation in different ethnic backgrounds.10
Skin barrier dysfunction
When the skin barrier is disrupted, it causes Th2 cells to produce IL-31. This, in turn, triggers eosinophils to release various cytokines. These cytokines activate Th2 cells, leading to a cycle of production of IL-4 and IL-13. Th22 and Th1 cytokines, IL-22, and IFN-γ also play an important role in skin barrier dysfunction.1
The clinical impact of these pathways on patients with AD is not fully understood.
Th2, Th22, and Th1 cytokines shown are not exhaustive; other cytokines in the Th2, Th22, and Th1 classes are not shown and there is increasing attention regarding endotype presentation in different ethnic backgrounds.10
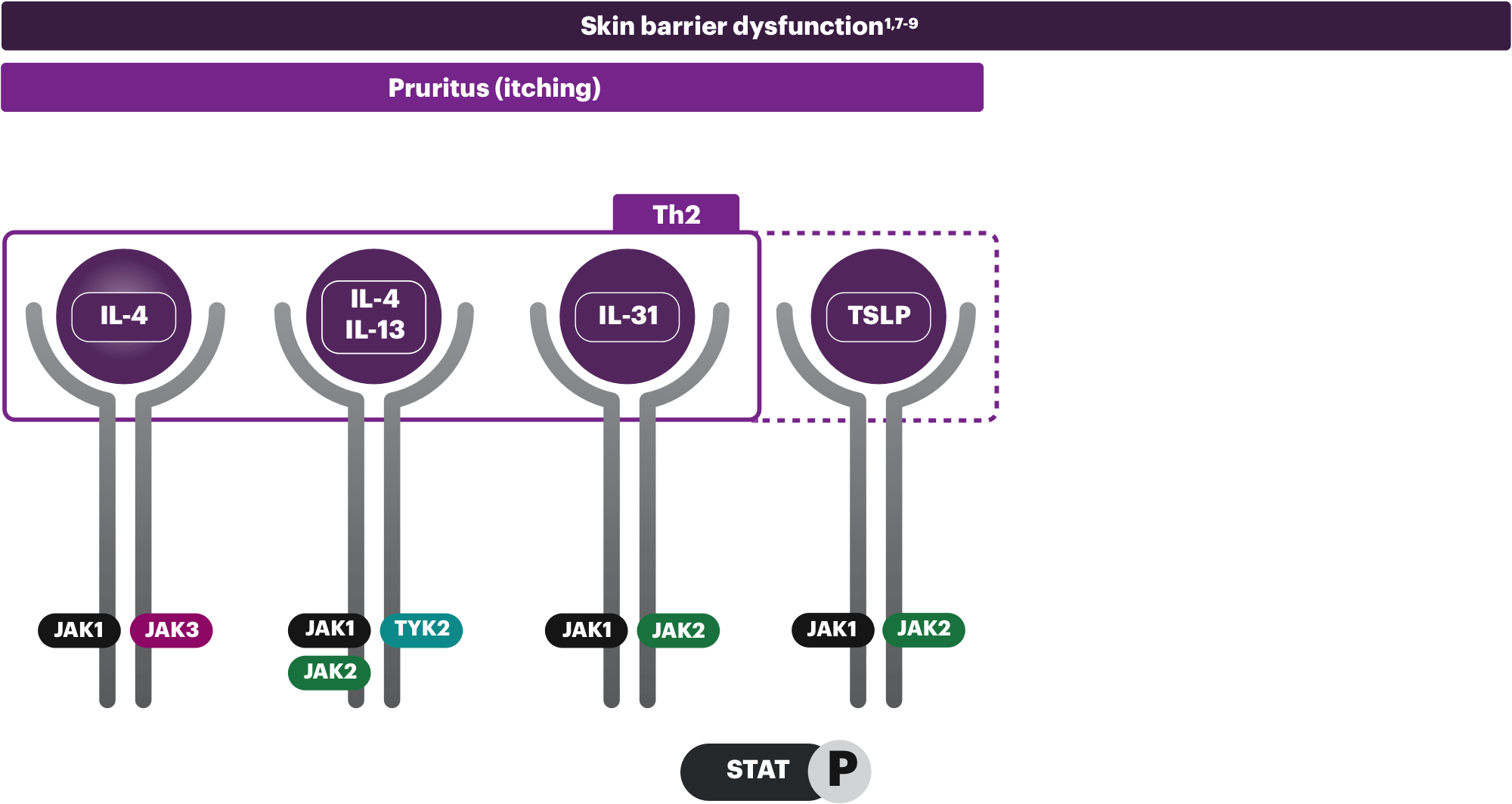
Pruritus (itching)
Cytokines signal through multiple pathways, but IL-31 (the "itch" cytokine) and TSLP are the primary drivers in the development of pruritus. In addition to IL-31, IL-4, and IL-13 activate sensory neurons to contribute to histamine-independent itch.1,8
The clinical impact of these pathways on patients with AD is not fully understood.
Th2, Th22, and Th1 cytokines shown are not exhaustive; other cytokines in the Th2, Th22, and Th1 classes are not shown and there is increasing attention regarding endotype presentation in different ethnic backgrounds.10
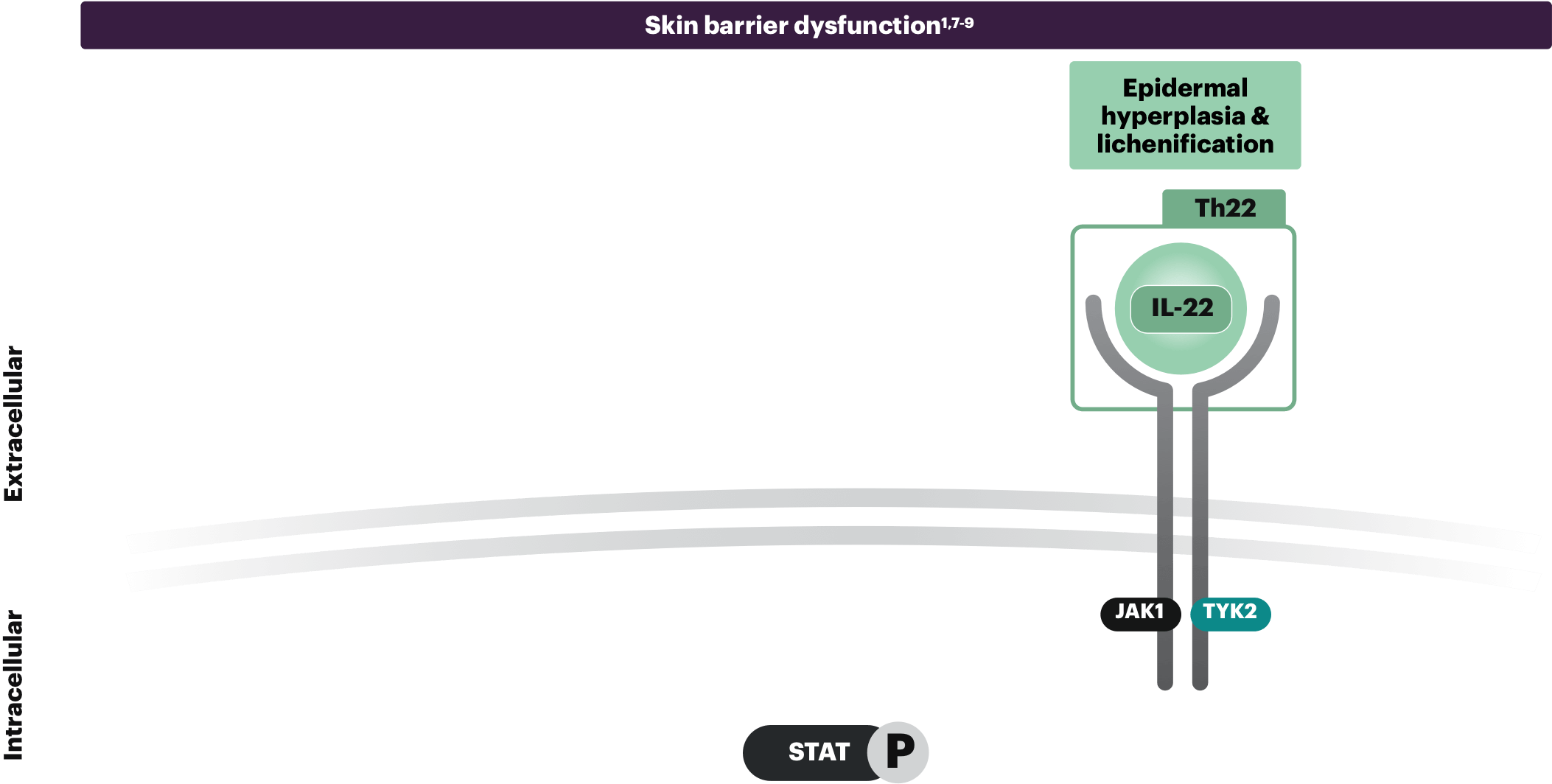
Epidermal hyperplasia & lichenification
IL-22 is elevated in AD lesions and is associated with epidermal thickening and skin barrier disruption. IL-22 is thought to signal through JAK1 and TYK2, boosting the expression of proinflammatory genes in keratinocytes, contributing to epidermal hyperplasia and lichenification.1,9
The clinical impact of these pathways on patients with AD is not fully understood.
Th2, Th22, and Th1 cytokines shown are not exhaustive; other cytokines in the Th2, Th22, and Th1 classes are not shown and there is increasing attention regarding endotype presentation in different ethnic backgrounds.10
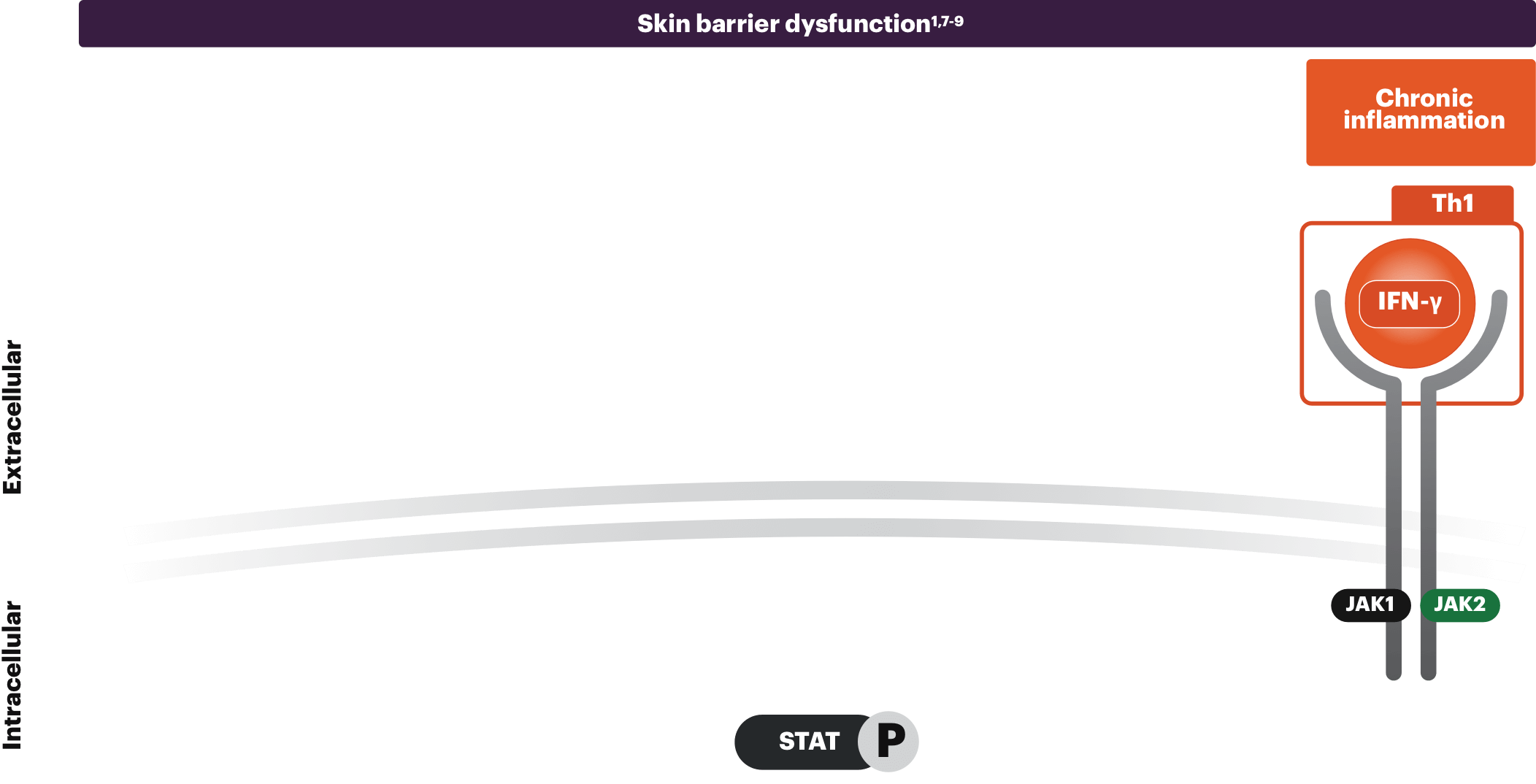
Chronic inflammation
IFN-γ is a Th1 cytokine secreted during the chronic stages of AD. This increase in IFN-γ levels contributes to the inflammatory response and causes keratinocyte apoptosis, all playing a part in the progressive relationship of itch and scratch.1
See why it's so important to break the itch-scratch cycle
AD is often defined by chronic itch that leads to scratching. The act of scratching is a reflexive response that only provides temporary relief, and can result in damaged skin and increased inflammation. This may further exacerbate and continue the itch-scratch cycle of AD.8
IFN=interferon; IL=interleukin; JAK=Janus kinase; TH=T-helper cell; TSLP=thymic stromal lymphopoietin; TYK=tyrosine kinase.
REFERENCES:
- Guttman-Yassky E, Irvine AD, Brunner PM, et al. The role of Janus kinase signaling in the pathology of atopic dermatitis. J Allergy Clin Immunol. 2023;152(6):1394-1404. doi:10.1016/j.jaci.2023.07.010
- Brunner PM, Leung DYM, Guttman-Yassky E. Immunologic, microbial, and epithelial interactions in atopic dermatitis. Ann Allergy Asthma Immunol. 2018;120(1):34-41. doi:10.1016/j.anai.2017.09.055
- Leung DYM, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014;134(4):769-779. doi:10.1016/j.jaci.2014.08.008
- Peng W, Novak N. Pathogenesis of atopic dermatitis. Clin Exp Allergy. 2015;45(3):566-574. doi:10.1111/cea.12495
- Kwatra SG, Misery L, Clibborn C, Steinhoff M. Molecular and cellular mechanisms of itch and pain in atopic dermatitis and implications for novel therapeutics. Clin Transl Immunology. 2022;11(5):e1390. doi:10.1002/cti2.1390
- Noda S, Krueger JG, Guttman-Yassky E. The translational revolution and use of biologics in patients with inflammatory skin diseases. J Allergy Clin Immunol. 2015;135(2):324-336. doi:10.1016/j.jaci.2014.11.015
- Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140(3):633-643. doi:10.1016/j.jaci.2017.07.006
- Legat FJ. Itch in atopic dermatitis - what is new? Front Med (Lausanne). 2021;8:644760. doi:10.3389/fmed.2021.644760
- Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol. 2019;10:2342. doi:10.3389/fimmu.2019.02342
- Sroka-Tomaszewska J, Trzeciak M. Molecular mechanisms of atopic dermatitis pathogenesis. Int J Mol Sci. 2021;22(8):4130. doi:10.3390/ijms22084130